Jason Crain
Accelerating Inhibitor Discovery for Multiple SARS-CoV-2 Targets with a Single, Sequence-Guided Deep Generative Framework
Apr 19, 2022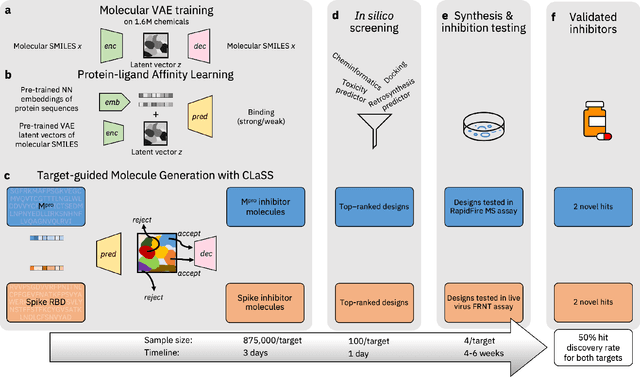

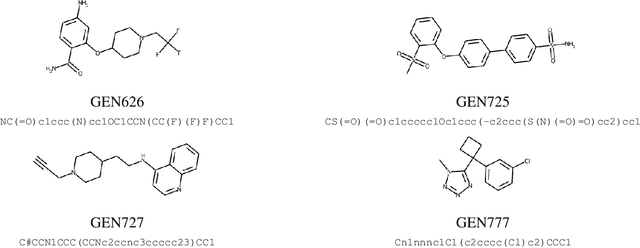

Abstract:The COVID-19 pandemic has highlighted the urgency for developing more efficient molecular discovery pathways. As exhaustive exploration of the vast chemical space is infeasible, discovering novel inhibitor molecules for emerging drug-target proteins is challenging, particularly for targets with unknown structure or ligands. We demonstrate the broad utility of a single deep generative framework toward discovering novel drug-like inhibitor molecules against two distinct SARS-CoV-2 targets -- the main protease (Mpro) and the receptor binding domain (RBD) of the spike protein. To perform target-aware design, the framework employs a target sequence-conditioned sampling of novel molecules from a generative model. Micromolar-level in vitro inhibition was observed for two candidates (out of four synthesized) for each target. The most potent spike RBD inhibitor also emerged as a rare non-covalent antiviral with broad-spectrum activity against several SARS-CoV-2 variants in live virus neutralization assays. These results show a broadly deployable machine intelligence framework can accelerate hit discovery across different emerging drug-targets.
Accelerating Antimicrobial Discovery with Controllable Deep Generative Models and Molecular Dynamics
May 22, 2020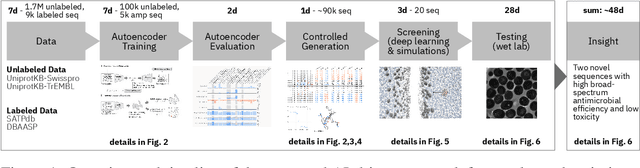
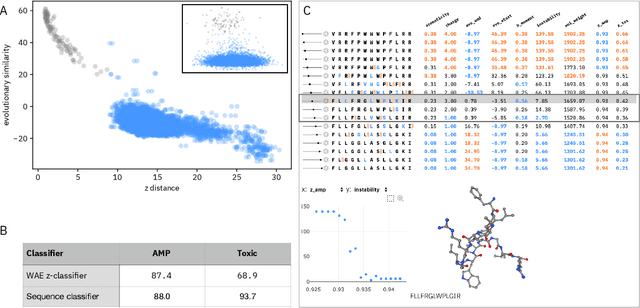
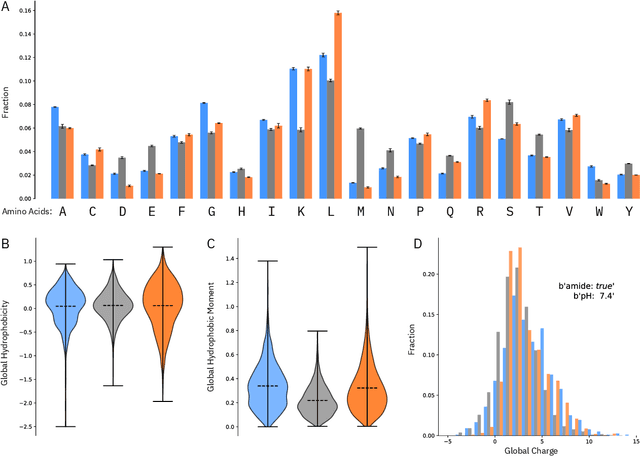
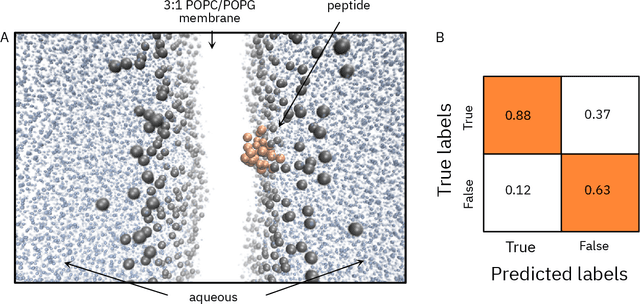
Abstract:De novo therapeutic design is challenged by a vast chemical repertoire and multiple constraints such as high broad-spectrum potency and low toxicity. We propose CLaSS (Controlled Latent attribute Space Sampling) - a novel and efficient computational method for attribute-controlled generation of molecules, which leverages guidance from classifiers trained on an informative latent space of molecules modeled using a deep generative autoencoder. We further screen the generated molecules by using a set of deep learning classifiers in conjunction with novel physicochemical features derived from high-throughput molecular simulations. The proposed approach is employed for designing non-toxic antimicrobial peptides (AMPs) with strong broad-spectrum potency, which are emerging drug candidates for tackling antibiotic resistance. Synthesis and wet lab testing of only twenty designed sequences identified two novel and minimalist AMPs with high potency against diverse Gram-positive and Gram-negative pathogens, including the hard-to-treat multidrug-resistant K. pneumoniae, as well as low in vitro and in vivo toxicity. The proposed approach thus presents a viable path for faster discovery of potent and selective broad-spectrum antimicrobials with a higher success rate than state-of-the-art methods.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge